
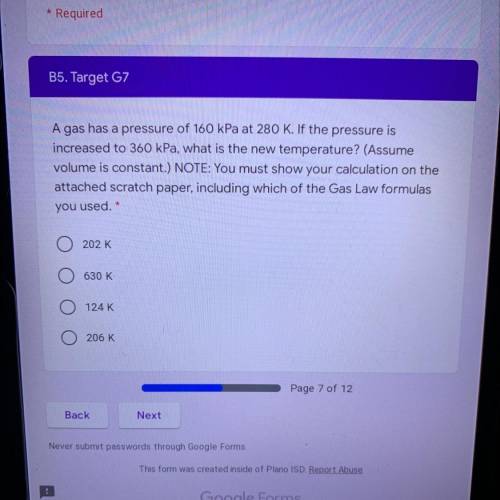
A gas has a pressure of 160 kPa at 280 K. If the pressure is
increased to 360 kPa, what is the new temperature? (Assume
volume is constant.) NOTE: You must show your calculation on the
attached scratch paper, including which of the Gas Law formulas
you used. *
A. 202 K
B. 630 K
C. 124 K
D. 206 K

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 16:40, raincalderxn
Which statement is true about market economies? government goals drive business decisions. people have the freedom to choose their jobs. several are market economies vist around the world
Answers: 2
You know the right answer?
A gas has a pressure of 160 kPa at 280 K. If the pressure is
increased to 360 kPa, what is the new...
Questions in other subjects:


Mathematics, 10.12.2020 21:30








English, 10.12.2020 21:30



