
Chemistry, 23.05.2021 07:50 autumng7518
in this assignment you will write and balance a chemical equation. then you will use a table of enthalpy values to calculate the energy charge in the reaction. next, you will create a model of the energy change in the reaction. finally, you will write an explanation that describes the energy change in the reaction
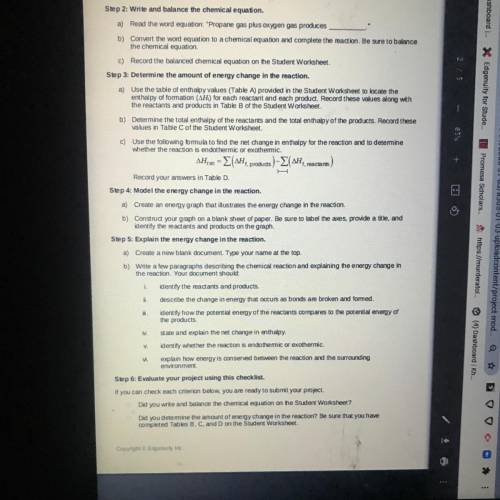

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, microwave13016
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1


Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
in this assignment you will write and balance a chemical equation. then you will use a table of enth...
Questions in other subjects:

English, 07.11.2020 21:20


English, 07.11.2020 21:20

Social Studies, 07.11.2020 21:20

Mathematics, 07.11.2020 21:20

Mathematics, 07.11.2020 21:20


Advanced Placement (AP), 07.11.2020 21:20




