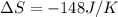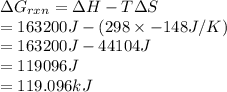AHM

Chemistry, 22.05.2021 20:00 bankscorneliuso39
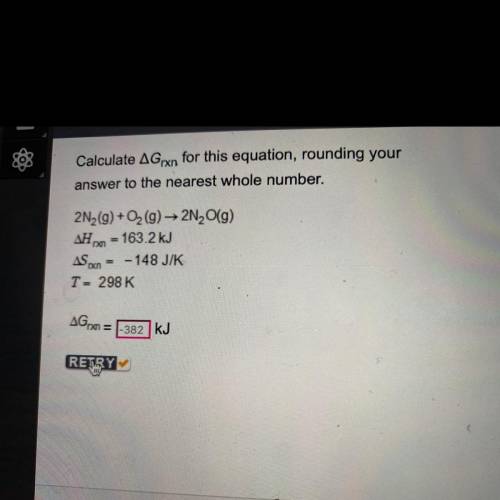
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
AHM
2N2(g) + O2(g) → 2N (9)
163.2 kJ
ASX = -148 J/K
T = 298 K
AGO
kJ

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 08:00, kendrawalraven
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3

Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
AHM
AHM
Questions in other subjects:




Mathematics, 20.02.2021 14:30


Biology, 20.02.2021 14:30




Biology, 20.02.2021 14:30

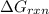 for given equation is 119.096 kJ.
for given equation is 119.096 kJ. 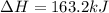 (1 kJ = 1000 J) = 163200 J
(1 kJ = 1000 J) = 163200 J