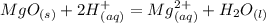Chemistry, 22.05.2021 18:10 mistycascaden
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Please let me know how you worked it out, thankyou!!

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

You know the right answer?
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Questions in other subjects:




Mathematics, 09.03.2021 01:00

Health, 09.03.2021 01:00



Mathematics, 09.03.2021 01:00

English, 09.03.2021 01:00