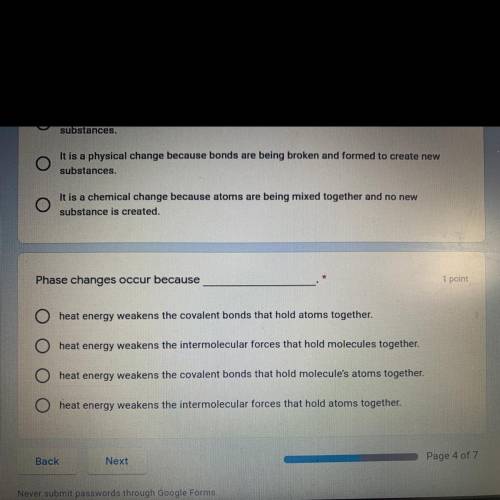
Phase changes occur because_
...

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, iloveballet1857
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Questions in other subjects:


History, 16.01.2020 10:31




Chemistry, 16.01.2020 10:31