Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 11:40, missmontgomery21
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
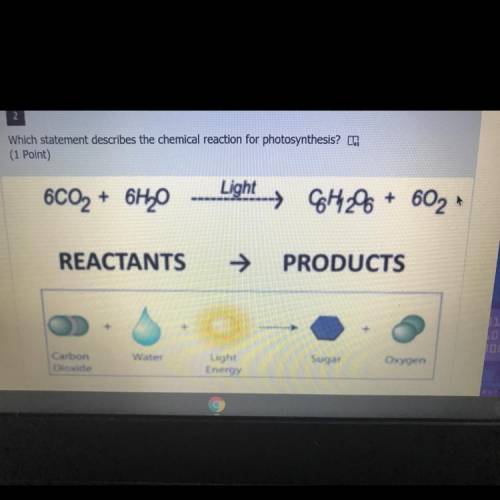
A.) the reaction is exothermic and absorbs energy
B.) the reaction is exothermic and releases energ...
Questions in other subjects:


Mathematics, 16.12.2021 22:00

Biology, 16.12.2021 22:00

English, 16.12.2021 22:00

Social Studies, 16.12.2021 22:00



Arts, 16.12.2021 22:00

Mathematics, 16.12.2021 22:00

Spanish, 16.12.2021 22:00