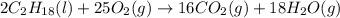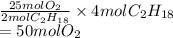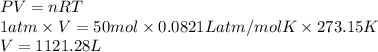Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, officialrogerfp3gf2s
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxyge...
Questions in other subjects:

English, 09.12.2021 18:00


History, 09.12.2021 18:00

Computers and Technology, 09.12.2021 18:00



SAT, 09.12.2021 18:00


Mathematics, 09.12.2021 18:00