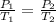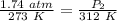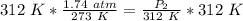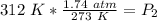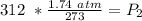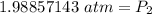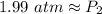Chemistry, 21.05.2021 06:20 themaster66644
A gas has a pressure of 1.74 atm when the temperature is 237K. The gas is then
heated until the temperature measures 312K. What will be the new pressure? *
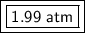
2.29 atm
1.32 atm
O 128662.56 atm

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1


Chemistry, 23.06.2019 07:30, 22emilyl530
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
A gas has a pressure of 1.74 atm when the temperature is 237K. The gas is then
heated until the tem...
Questions in other subjects:

Mathematics, 28.11.2019 20:31

Mathematics, 28.11.2019 20:31


Computers and Technology, 28.11.2019 20:31


Mathematics, 28.11.2019 20:31

History, 28.11.2019 20:31