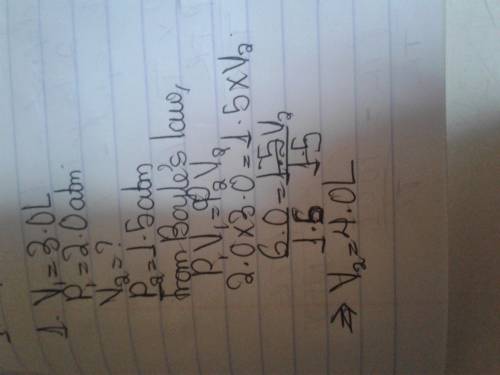Chemistry, 20.05.2021 22:40 sheram2010
The volume of a gas at 2.0 atm is 3.0 L. What is the volume of the gas at 1.5 atm at the same temperature?
0.50 L
1.0 L
1.5 L
4.0 L

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
The volume of a gas at 2.0 atm is 3.0 L. What is the volume of the gas at 1.5 atm at the same temper...
Questions in other subjects:


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01