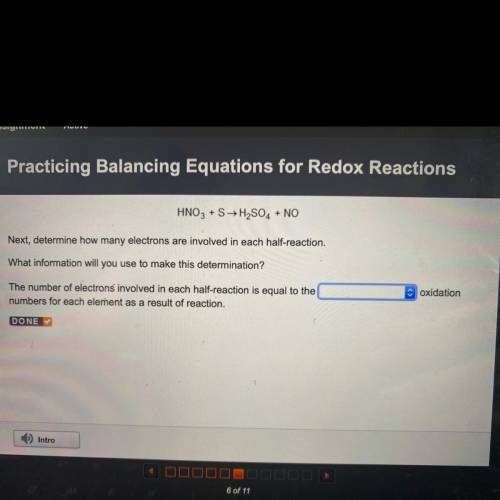
HNO3 +S → H2SO4 + NO
Next, determine how many electrons are involved in each half-reaction.
W...

Chemistry, 20.05.2021 22:20 recon12759
HNO3 +S → H2SO4 + NO
Next, determine how many electrons are involved in each half-reaction.
What information will you use to make this determination?
c oxidation
The number of electrons involved in each half-reaction is equal to the
numbers for each element as a result of reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 12.09.2021 04:40


SAT, 12.09.2021 04:40

World Languages, 12.09.2021 04:40


English, 12.09.2021 04:40

Mathematics, 12.09.2021 04:40



English, 12.09.2021 04:40



