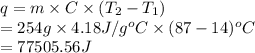Chemistry, 20.05.2021 19:40 nicholasferrell
The initial temperature of the water in a constant-pressure calorimeter is
14°C. A reaction takes place in the calorimeter, and the temperature rises
to 87°C. The calorimeter contains 254 g of water, which has a specific heat
of 4.18 J/(g.°C). Calculate the enthalpy change during this reaction. *

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
The initial temperature of the water in a constant-pressure calorimeter is
14°C. A reaction takes p...
Questions in other subjects:

Mathematics, 04.12.2020 02:00


Biology, 04.12.2020 02:00

Health, 04.12.2020 02:00





Arts, 04.12.2020 02:00

Mathematics, 04.12.2020 02:00

 ,
, 


 = initial temperature
= initial temperature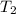 = final temperature
= final temperature