

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:30, kylee65
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
The pOH of a solution of KOH is 11.30. What is the [H*] for this solution?...
Questions in other subjects:

Mathematics, 16.04.2021 05:20


Mathematics, 16.04.2021 05:20




Mathematics, 16.04.2021 05:20

Mathematics, 16.04.2021 05:20


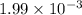 .
.![pH = - log [H^{+}]](/tpl/images/1336/4302/d6b92.png)
![pH = -log [H^{+}]\\2.7 = -log [H^{+}]\\conc. of H^{+} = 1.99 \times 10^{-3}](/tpl/images/1336/4302/3ee68.png)



