
Chemistry, 19.05.2021 18:00 jazzyjaz2003
27.5 mL of a 0.235 M potassium hydroxide solution is required to completely react with 35.0 mL of a sulfuric acid solution. Provide the balanced chemical equation for this reaction and determine the concentration of the sulfuric acid solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
27.5 mL of a 0.235 M potassium hydroxide solution is required to completely react with 35.0 mL of a...
Questions in other subjects:

Computers and Technology, 31.01.2021 17:40





Mathematics, 31.01.2021 17:40

Mathematics, 31.01.2021 17:40

Mathematics, 31.01.2021 17:40


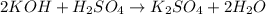 and the concentration of the sulfuric acid solution is 0.184 M.
and the concentration of the sulfuric acid solution is 0.184 M.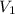 = 27.5 mL,
= 27.5 mL, 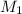 = 0.235 M
= 0.235 M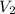 = 35.0 mL,
= 35.0 mL, 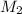 = ?
= ?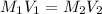
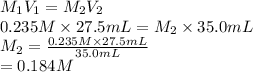

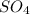 = 1O = 1
= 1O = 1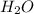 by 2. Hence, the equation can be rewritten as follows.
by 2. Hence, the equation can be rewritten as follows.

