
Chemistry, 19.05.2021 15:00 Blahdjwj108
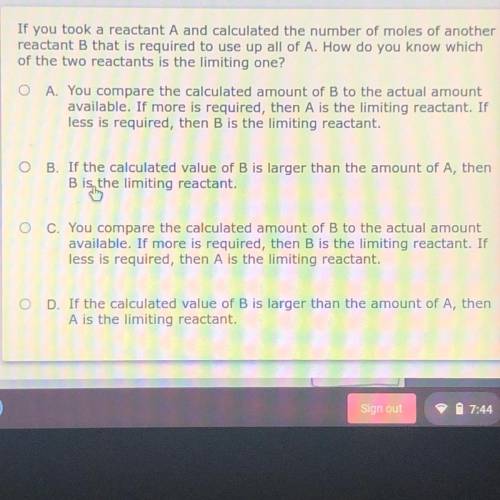
If you took a reactant A and calculated the number of moles of another
reactant B that is required to use up all of A. How do you know which
of the two reactants is the limiting one?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 23.06.2019 10:30, GiuliAzevedo
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2

Chemistry, 23.06.2019 10:30, genyjoannerubiera
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2
You know the right answer?
If you took a reactant A and calculated the number of moles of another
reactant B that is required...
Questions in other subjects:

Mathematics, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01



Mathematics, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01

English, 22.10.2020 04:01

English, 22.10.2020 04:01



