
Chemistry, 19.05.2021 01:00 estefaniapenalo
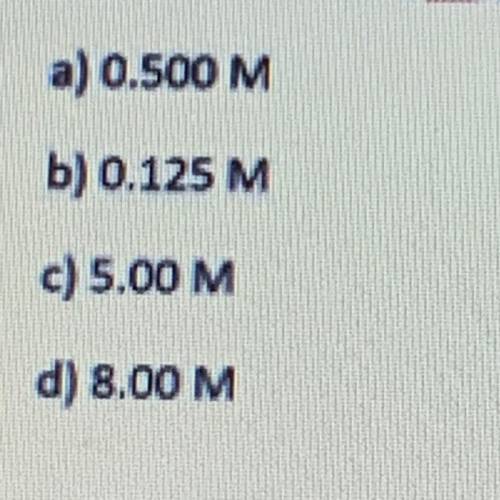
A volume of 20.0 of 0.250 M neutralizes a 40.0 sample of KOH. What is the concentration of кон? HCl(aq)+KOH(aq) KCl(aq)+H 2 O(l)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
A volume of 20.0 of 0.250 M neutralizes a 40.0 sample of KOH. What is the concentration of кон? HCl(...
Questions in other subjects:

Mathematics, 12.02.2020 03:10



Mathematics, 12.02.2020 03:10




History, 12.02.2020 03:10


English, 12.02.2020 03:10



