
Chemistry, 18.05.2021 20:30 Nismo3501037
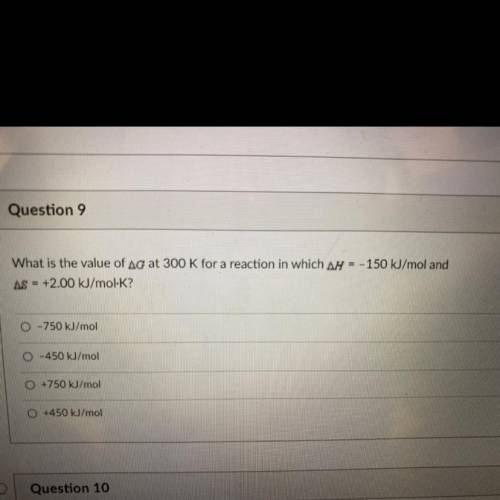
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and AS = +2.00 kJ/mol-K?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and
AS = +2.00 kJ/mol-K?...
Questions in other subjects:




History, 16.10.2021 18:30


History, 16.10.2021 18:30



History, 16.10.2021 18:30

Social Studies, 16.10.2021 18:40



