
Chemistry, 18.05.2021 19:20 catzdatbloadd
It is desired that an acetic acid sodium acetate buffered solution have a pH of 5.27. You have a solution that has an acetic acid concentration of 0.01 M. What molarity of sodium acetate will you need to add to the solution, given that the pKa of acetic acid is 4.74

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, hannahkelly3618
How many moles are equivalent to 55.5g of nano3
Answers: 1

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
It is desired that an acetic acid sodium acetate buffered solution have a pH of 5.27. You have a sol...
Questions in other subjects:


Mathematics, 11.03.2020 02:44


Mathematics, 11.03.2020 02:44






![pH = pK_a + \log \frac{[A^-]}{[HA]}](/tpl/images/1331/4715/1110c.png)
![[Ac^-]](/tpl/images/1331/4715/fda84.png) = Concentration of the conjugate base
= Concentration of the conjugate base 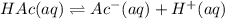
![[Ac^-] = ?](/tpl/images/1331/4715/57252.png)
![5.27= 4.74 + \log \frac{[Ac^-]}{[0.01 M]}](/tpl/images/1331/4715/91873.png)
![[Ac^-]=0.0339 M\approx 0.034M](/tpl/images/1331/4715/e0d23.png)
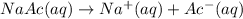
![[Ac^-]=[Na^+]=[NaAc]= 0.034M](/tpl/images/1331/4715/0e140.png)



