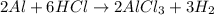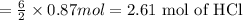Chemistry, 18.05.2021 19:20 raymondleggett44
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen. Write a balanced equation for the reaction and calculate the number of moles of hydrochloric acid required to react with 0.87 mole of aluminum.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen. Write a balanced e...
Questions in other subjects:


History, 26.03.2021 23:50



English, 26.03.2021 23:50


Biology, 27.03.2021 01:00

Mathematics, 27.03.2021 01:00


Mathematics, 27.03.2021 01:00