
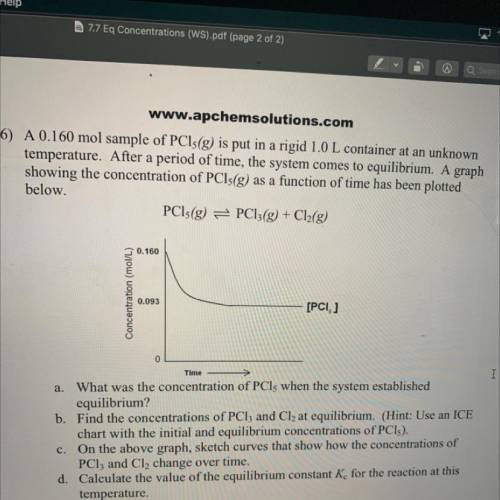
6) A 0.160 mol sample of PCls(g) is put in a rigid 1.0 L container at an unknown
temperature. After a period of time, the system comes to equilibrium. A graph
showing the concentration of PCls(g) as a function of time has been plotted
below.
PCls(g) = PC13(g) + Cl2(g)
0.160
Concentration (mol/L)
0.093
[PCI, ]
I
Time
a. What was the concentration of PCls when the system established
equilibrium?
b. Find the concentrations of PCl3 and Cl2 at equilibrium. (Hint: Use an ICE
chart with the initial and equilibrium concentrations of PCI).
c. On the above graph, sketch curves that show how the concentrations of
PClz and Cl, change over time.
d. Calculate the value of the equilibrium constant K for the reaction at this
temperature.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 17:30, destineysarah
98 points you will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. before you conduct your experiment, you need to form a hypothesis. a hypothesis is a prediction of what you think will happen in the experiment. the hypothesis is a statement that describes “if” a certain set of circumstances are present “then” there will be a specific result that will occur. record your hypothesis here: record the results from step one of the experiment (dropping the objects in the air): first trial: second trial: third trial: record the results from step two of the experiment (dropping the objects in a vacuum): first trial: second trial: third trial: did the experiment support your hypothesis? using the data from your experiment, describe why you believe your hypothesis was either proven or disproven. what forces were acting on the objects dropped in the air? what force was acting on the objects dropped in the vacuum? part two: comparing forces choose two forces and compare and contrast these forces. you must provide two ways that they are alike and two ways that they are different. you may make a list, write in paragraph form, or make a chart. choose two forces and compare and contrast these forces. these must be different forces than used in the prior question. provide two ways that they are similar and two ways that they are different. you may make a list, write it out, or make a chart.
Answers: 3
You know the right answer?
6) A 0.160 mol sample of PCls(g) is put in a rigid 1.0 L container at an unknown
temperature. After...
Questions in other subjects:


Mathematics, 01.11.2020 01:20


Social Studies, 01.11.2020 01:20

Mathematics, 01.11.2020 01:20

Mathematics, 01.11.2020 01:20

Biology, 01.11.2020 01:20



Computers and Technology, 01.11.2020 01:20



