0 0
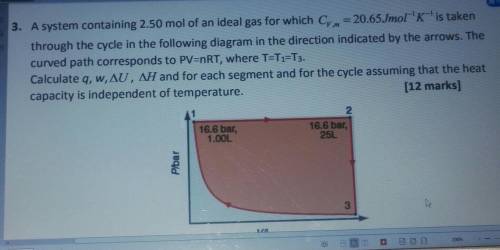
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
t...

Chemistry, 17.05.2021 23:10 pablohc200021
0 0
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
through the cycle in the following diagram in the direction indicated by the arrows. The
curved path corresponds to PV=nRT, where T=T1=T3.
Calculate q, w, AU, AH and for each segment and for the cycle assuming that the heat
capacity is independent of temperature.
(12 marks]
2.
16.6 bar,
1.00L
16.6 bar,
25L
Plbar
یا
3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Questions in other subjects:






History, 29.06.2019 05:00




Mathematics, 29.06.2019 05:00



