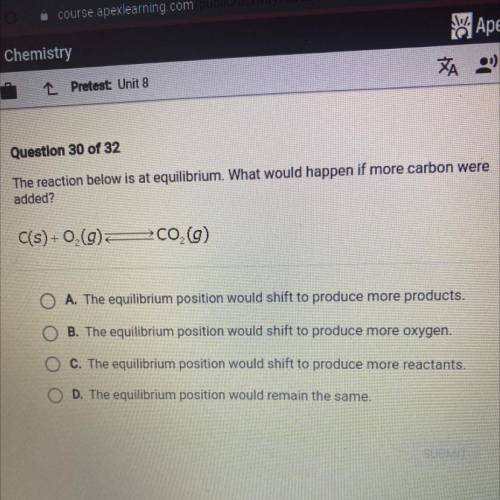
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9...

The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9)
700 (9)
A. The equilibrium position would shift to produce more products.
B. The equilibrium position would shift to produce more oxygen.
C. The equilibrium position would shift to produce more reactants.
D. The equilibrium position would remain the same.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1



Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Questions in other subjects:



Mathematics, 26.10.2021 19:40

Mathematics, 26.10.2021 19:40

Social Studies, 26.10.2021 19:40







