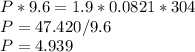Chemistry, 17.05.2021 17:40 loveyeti106838
At 304 K, a 9.6 L tank contains 1.9 moles of H2 gas under an unknown pressure in atm. What is the pressure of the gas in the tank? (R = 0.0821 L*atm/mol*K)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
At 304 K, a 9.6 L tank contains 1.9 moles of H2 gas under an unknown pressure in atm. What is the pr...
Questions in other subjects:

Mathematics, 19.08.2019 15:00


Mathematics, 19.08.2019 15:00

English, 19.08.2019 15:00



Chemistry, 19.08.2019 15:00

Social Studies, 19.08.2019 15:00


Social Studies, 19.08.2019 15:00