Name:
Based on the equation:
10 Na +
2 NaNO, →
6 Na, O + N2
1.
How ma...

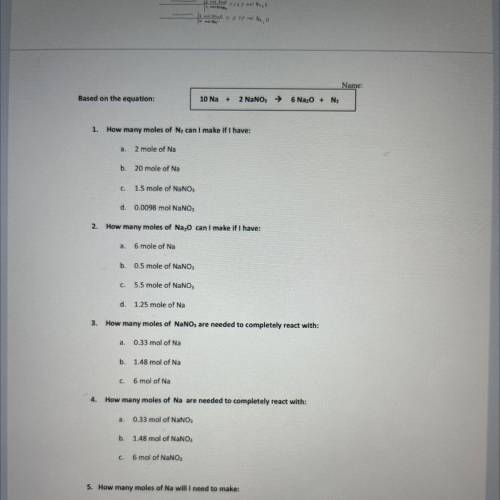
Name:
Based on the equation:
10 Na +
2 NaNO, →
6 Na, O + N2
1.
How many moles of N, can I make if I have:
a.
2 mole of Na
b.
20 mole of Na
C.
1.5 mole of NaNO,
d. 0.0098 mol NaNO3
2. How many moles of Na20 can I make if I have:
a
6 mole of Na
b.
0.5 mole of NaNO3
c. 5.5 mole of NaNO;
d. 1.25 mole of Na
3. How many moles of NaNO, are needed to completely react with:
a.
0.33 mol of Na
b. 1.48 mol of Na
c
6 mol of Na
4. How many moles of Na are needed to completely react with:
0.33 mol of NaNO;
b. 1.48 mol of NaNO3
c.
6 mol of NaNO,
5. How many moles of Na will I need to make:
a.
8 mol of Ne
b.
3 mol of N
с.
15 mol of Na20

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Questions in other subjects:


Biology, 31.03.2020 22:43

Mathematics, 31.03.2020 22:43

Mathematics, 31.03.2020 22:43



Mathematics, 31.03.2020 22:43



Mathematics, 31.03.2020 22:43



