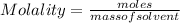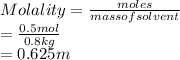Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 16:00, jakebuttone
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a. no b. n2o4 c. no2 d. n4o8
Answers: 1

Chemistry, 23.06.2019 22:30, catchonyet
Identify the limiting reagent and the volume of co2 formed when 11 l cs2 reacts with 18 l o2 to produce co2 gas and so2 gas at stp options: cs2; 5.5l co2 o2; 6.0 l co2 cs2; 11 l co2 o2; 27l co2
Answers: 1
You know the right answer?
What is the molality of an acetic acid solution that contains 0.500 mol of HC2H3O2 (molar mass: 60g/...
Questions in other subjects:



Mathematics, 22.06.2019 03:30


Mathematics, 22.06.2019 03:30



Advanced Placement (AP), 22.06.2019 03:30