Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1



Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
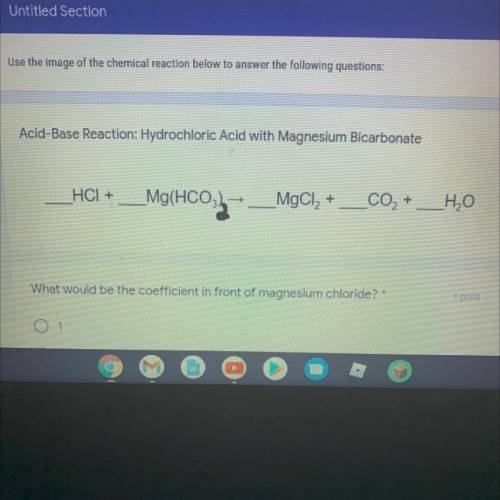
Acid-Base Reaction: Hydrochloric Acid with Magnesium Bicarbonate
__HCI + _Mg(HCO3)2 —> _MgCl2 +...
Questions in other subjects:

Health, 23.02.2021 23:30

Business, 23.02.2021 23:30

Mathematics, 23.02.2021 23:30


Social Studies, 23.02.2021 23:30

Mathematics, 23.02.2021 23:30

Mathematics, 23.02.2021 23:30



Social Studies, 23.02.2021 23:30