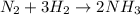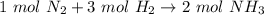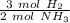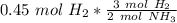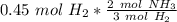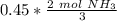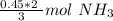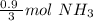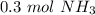Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3


Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
How many moles of NH3 are produced when 0.45 moles of H2 gas react
with N2 gas? N2 + 3H2 -->2 NH...
Questions in other subjects:

History, 25.11.2020 20:20



Social Studies, 25.11.2020 20:20



Physics, 25.11.2020 20:20



Chemistry, 25.11.2020 20:20