
Chemistry, 15.05.2021 05:10 ninaaforever
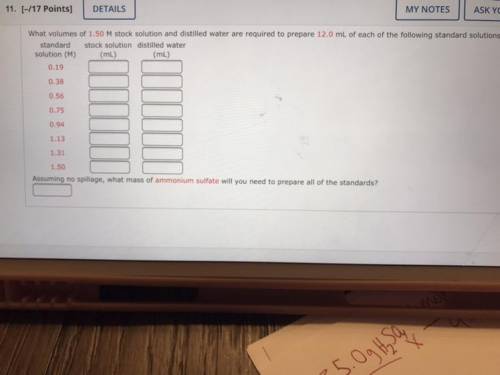
What volumes of 1.50 M stock solution and distilled water are required to prepare 12.0 mL of each of the following standard solutions:
standard stock solution distilled water
solution (M)
(mL)
0.19
(mL)
0.38
0.56
0.75
0.94
1.13
1.31
1.50
Assuming no spillage, what mass of ammonium sulfate will you need to prepare all of the standards?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
What volumes of 1.50 M stock solution and distilled water are required to prepare 12.0 mL of each of...
Questions in other subjects:

English, 30.09.2019 07:00

Mathematics, 30.09.2019 07:00




Mathematics, 30.09.2019 07:00



History, 30.09.2019 07:00



