Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1



Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
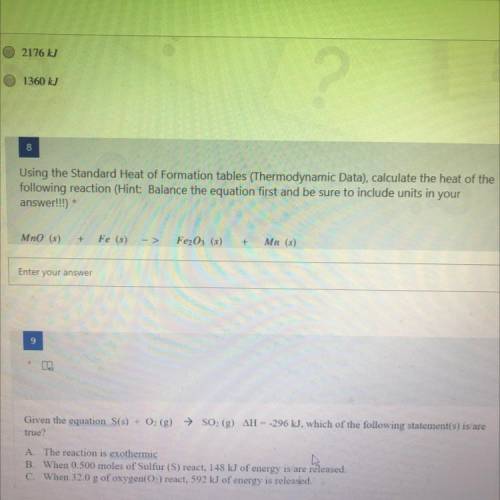
Using the Standard Heat of Formation tables (Thermodynamic Data), calculate the heat of the
followi...
Questions in other subjects:

Mathematics, 10.05.2021 08:00

Mathematics, 10.05.2021 08:00



Mathematics, 10.05.2021 08:00

History, 10.05.2021 08:00


Advanced Placement (AP), 10.05.2021 08:00