
Chemistry, 14.05.2021 23:20 saltyimps3
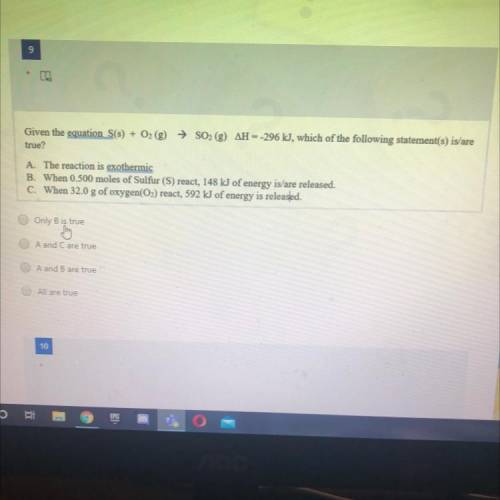
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are
true?
A. The reaction is exothermic
B. When 0.500 moles of Sulfur (S) react, 148 kJ of energy is/are released.
C. When 32.0 g of oxygen(O2) react, 592 kJ of energy is released.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1


Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are...
Questions in other subjects:



Biology, 24.04.2021 14:30

Mathematics, 24.04.2021 14:30

Mathematics, 24.04.2021 14:30




Mathematics, 24.04.2021 14:40

Mathematics, 24.04.2021 14:40



