
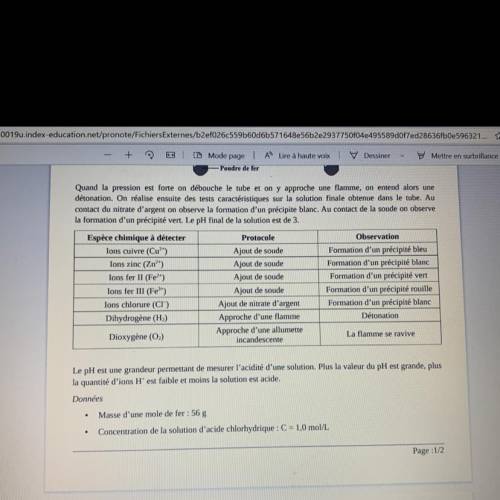
Quand la pression est forte on débouche le tube et on n’y approche une flamme on entend alors une détonation on réalise ensuite des tests caractéristiques sur la solution finale obtenue dans le tube au contact du nitrate d’argent on observe la formation d’un précipit blanc. au contact de la soude on observe la formation d’un précipité vert. le pH final de la solution est de 3.
Le pH est une grandeur permettant de mesurer l’acidité d’une solution plus la valeur du pH est grande plus la quantité d’ions H+ plus est faible et moins la solution est acide
Masse d’une mole de fer: 56g
Concentration de la solution d’acide chlorhydrique: C= 1,0mol/L
1-identifier les espèces chimiques présentes à la fin de la transformation
2-Écrire l’équation de réaction de cette transformation chimique et l’ajuster
3-déterminer les quantités de matière initiale de chaque réactif en mol
4 en déduire le réactif limitant

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3


Chemistry, 22.06.2019 07:20, rex1578
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
Quand la pression est forte on débouche le tube et on n’y approche une flamme on entend alors une dé...
Questions in other subjects:




Mathematics, 15.11.2020 05:00


History, 15.11.2020 05:00

English, 15.11.2020 05:00





