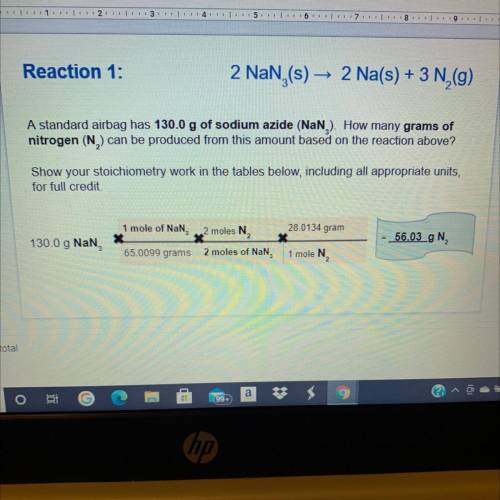
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of so...

Chemistry, 14.05.2021 05:40 lescoto9035
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of sodium produced from reaction 1, how many of grams of
nitrogen will also be produced in reaction 2?
+
Show your stoichiometry work in the tables below, including all appropriate units,
for full credit
gN
46 g Na
This slide is worth 2.5 pts total

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 01.12.2020 18:00


English, 01.12.2020 18:00

English, 01.12.2020 18:00

Mathematics, 01.12.2020 18:00

History, 01.12.2020 18:00

History, 01.12.2020 18:00

Social Studies, 01.12.2020 18:00




