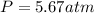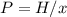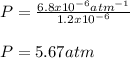Chemistry, 13.05.2021 23:10 mruffier6239
The mole fraction of helium in a saturated solution at 0°C is 1.2 x 10–6. Find the pressure of helium above the solution. Henry's law constant is 6.8 x 10–6 atm–1

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 04:00, Bassoonist
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
The mole fraction of helium in a saturated solution at 0°C is 1.2 x 10–6. Find the pressure of heliu...
Questions in other subjects:

Biology, 21.09.2019 22:50

Mathematics, 21.09.2019 22:50

Mathematics, 21.09.2019 22:50


History, 21.09.2019 22:50


Chemistry, 21.09.2019 22:50

Biology, 21.09.2019 22:50

Physics, 21.09.2019 22:50