
Chemistry, 13.05.2021 22:20 angieplasencia8
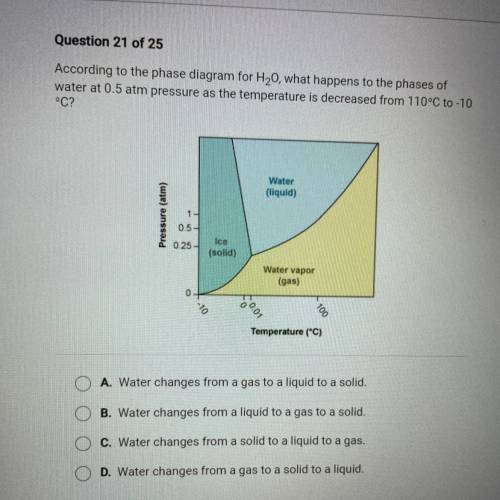
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as the temperature is decreased from 110°C to -10
°C?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, KayPink5723
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as...
Questions in other subjects:


English, 30.01.2020 08:02



Mathematics, 30.01.2020 08:02

Chemistry, 30.01.2020 08:02


Mathematics, 30.01.2020 08:02

Mathematics, 30.01.2020 08:02

English, 30.01.2020 08:02



