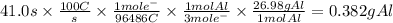Chemistry, 13.05.2021 20:50 naomicervero
In the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide Al2O3 dissolved in molten cryolite Na3AlF6, resulting in the reduction of the Al2O3 to pure aluminum. Suppose a current of 100.A is passed through a Hall-Heroult cell for 41.0 seconds. Calculate the mass of pure aluminum produced. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1


Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
In the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide...
Questions in other subjects:

Mathematics, 04.02.2020 23:49



Mathematics, 04.02.2020 23:49


Mathematics, 04.02.2020 23:49

World Languages, 04.02.2020 23:49