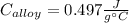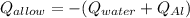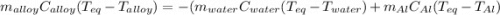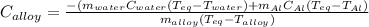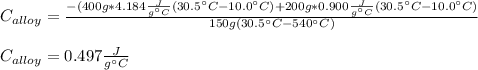Chemistry, 13.05.2021 20:40 ptanner706
A 0.150-kg sample of a metal alloy is heated at 540 Celsius an then plunged into a 0.400-kg of water at 10.0 Celsius, which is contained in a 0.200-kg aluminum calorimeter cup. The final temperature of the system is 30.5 Celsius. What is the specific heat of the metal alloy in J/Kg. Celsius

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

You know the right answer?
A 0.150-kg sample of a metal alloy is heated at 540 Celsius an then plunged into a 0.400-kg of water...
Questions in other subjects:


Mathematics, 05.06.2021 22:20






History, 05.06.2021 22:20


History, 05.06.2021 22:20