
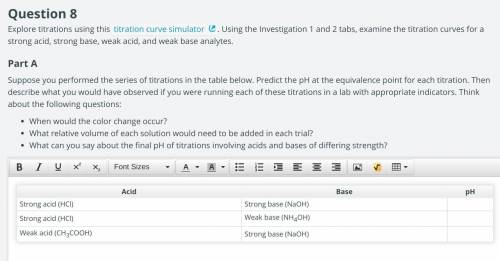
Suppose you performed the series of titrations in the table below. Predict the pH at the equivalence point for each titration. Then describe what you would have observed if you were running each of these titrations in a lab with appropriate indicators. ill give the link if needed

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

You know the right answer?
Suppose you performed the series of titrations in the table below. Predict the pH at the equivalence...
Questions in other subjects:






Social Studies, 22.12.2019 21:31

Social Studies, 22.12.2019 21:31

Mathematics, 22.12.2019 21:31

Health, 22.12.2019 21:31



