Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:20, zymikaa00
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
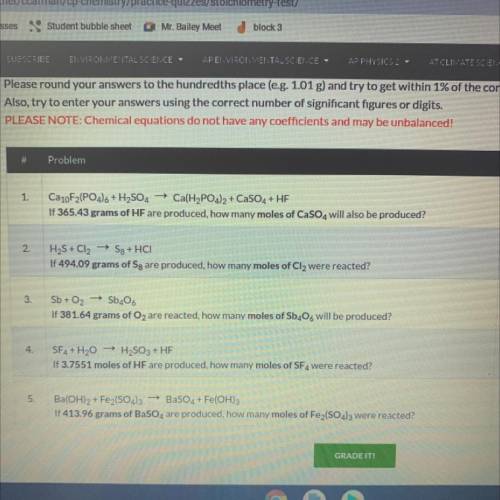
Ca10F2(PO4)6 + H2SO4 + Ca(H2PO4)2 + CaSO4 + HF
If 365.43 grams of HF are produced, how many moles o...
Questions in other subjects:

Biology, 07.07.2021 14:00


Social Studies, 07.07.2021 14:00


English, 07.07.2021 14:00

Physics, 07.07.2021 14:00

Engineering, 07.07.2021 14:00

English, 07.07.2021 14:00


Mathematics, 07.07.2021 14:00