
Chemistry, 12.05.2021 09:40 weckesserj9492
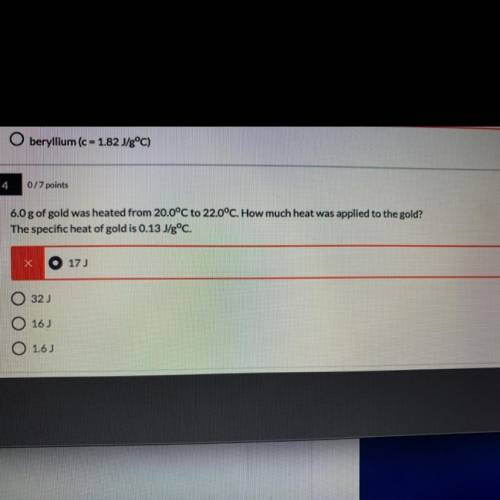
6.0 g of gold was heated from 20.0°C to 22.0°C. How much heat was applied to the gold?
The specific heat of gold is 0.13 J/gºC. (I need to explain why also )
A. 17J
B.32J
C.16J
D.1.6J

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
6.0 g of gold was heated from 20.0°C to 22.0°C. How much heat was applied to the gold?
The specific...
Questions in other subjects:



Mathematics, 13.10.2020 04:01

Social Studies, 13.10.2020 04:01

Social Studies, 13.10.2020 04:01


Mathematics, 13.10.2020 04:01


Mathematics, 13.10.2020 04:01

Social Studies, 13.10.2020 04:01



