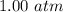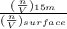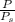Suppose Gabor, a scuba diver, is at a depth of 15m15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3g/cm3, which translates to an increase in pressure of 1.00 atmatm for every 10.0 mm of depth below the surface. Therefore, for example, at 10.0 mm, the net pressure is 2.00 atmatm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Suppose Gabor, a scuba diver, is at a depth of 15m15m. Assume that: The air pressure in his air trac...
Questions in other subjects:



Mathematics, 25.01.2021 05:10



Health, 25.01.2021 05:10

Mathematics, 25.01.2021 05:10

Health, 25.01.2021 05:10

Mathematics, 25.01.2021 05:10