
Chemistry, 12.05.2021 01:00 georgesarkes12
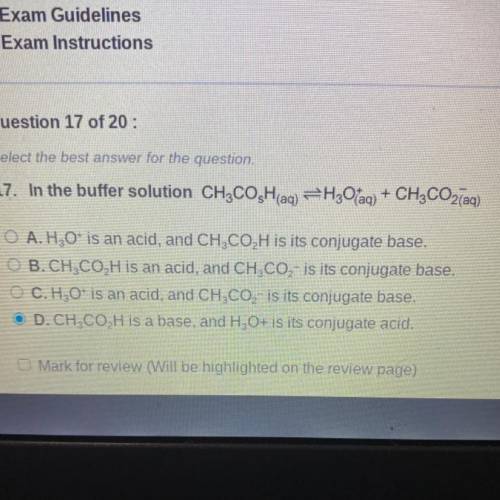
In the buffer solution CH3COsH(aq) -> H3O+(aq) + CH3CO2-(aq)
A. H3O+ is an acid, and CH3CO2H is its conjugate base.
B. CH3CO2H is an acid, and CH3CO2- is its conjugate base.
C. H3O+ is an acid, and CH3CO2- is its conjugate base.
D. CH3CO2H is a base, and H3O+ is its conjugate acid

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
In the buffer solution CH3COsH(aq) -> H3O+(aq) + CH3CO2-(aq)
A. H3O+ is an acid, and CH3CO2H i...
Questions in other subjects:



Mathematics, 31.03.2020 06:50

Business, 31.03.2020 06:50

Mathematics, 31.03.2020 06:50

English, 31.03.2020 06:50

Mathematics, 31.03.2020 06:50






