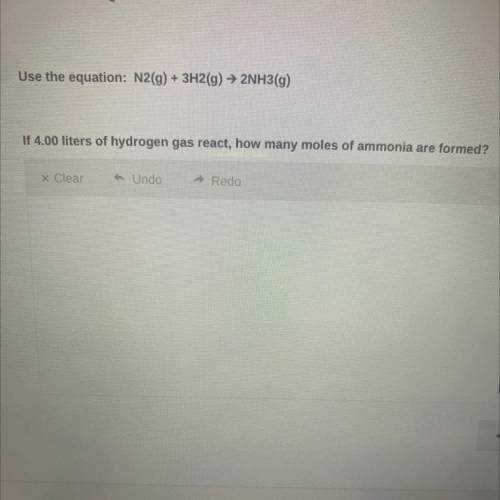
Use the equation:
N2(g) + 3H2(g) → 2NH3(g)
If 4.00 liters of hydrogen gas react, how ma...


Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

You know the right answer?
Questions in other subjects:


Biology, 19.07.2019 12:30

Mathematics, 19.07.2019 12:30

Geography, 19.07.2019 12:30




Mathematics, 19.07.2019 12:30


History, 19.07.2019 12:30