Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
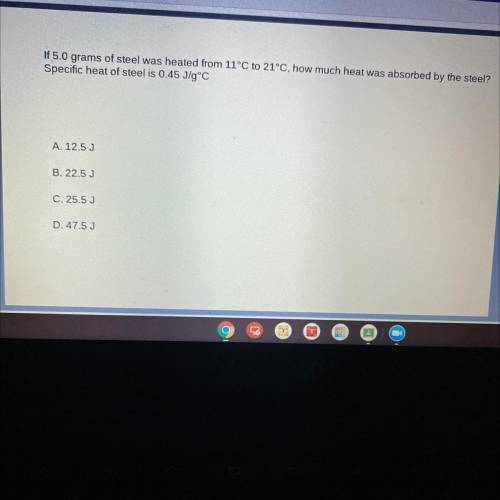
If 5.0 grams of steel was heated from 11°C to 21°C, how much heat was absorbed by the steel?
Spec...
Questions in other subjects:


History, 11.02.2020 19:20