
Chemistry, 11.05.2021 18:50 1r32tgy5hk7
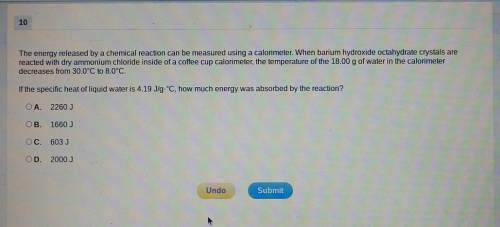
The energy released by a chemical reaction can be measured using a calorimeter. When barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00g of water in the calorimeter decreases from 30.0⁰C to 8.0⁰C. If the specific heat of liquid water is 4.19J/g.⁰C, how much energy was absorbed by the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2

Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
The energy released by a chemical reaction can be measured using a calorimeter. When barium hydroxid...
Questions in other subjects:

Biology, 11.06.2020 17:57


Mathematics, 11.06.2020 17:57



Mathematics, 11.06.2020 17:57

Mathematics, 11.06.2020 17:57

Mathematics, 11.06.2020 17:57


Chemistry, 11.06.2020 17:57



