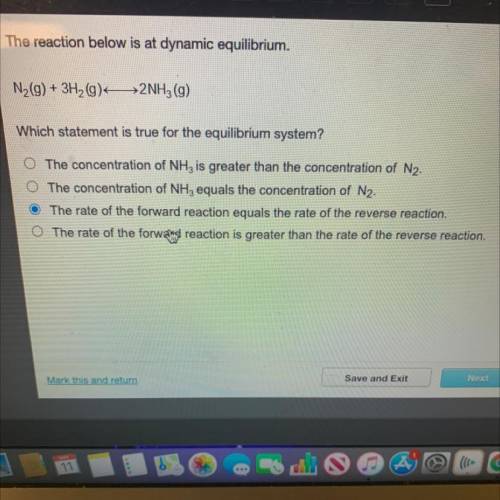
N(9) + 3H2(9) 2NH3 (9)
Which statement is true for the equilibrium system?
The concentration...

N(9) + 3H2(9) 2NH3 (9)
Which statement is true for the equilibrium system?
The concentration of NH, is greater than the concentration of N2.
O The concentration of NH3 equals the concentration of N2.
The rate of the forward reaction equals the rate of the reverse reaction.
O The rate of the forwald reaction is greater than the rate of the reverse reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1


Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 09:30, alexabdercmur
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10




English, 03.03.2021 18:10

English, 03.03.2021 18:10


Business, 03.03.2021 18:10



