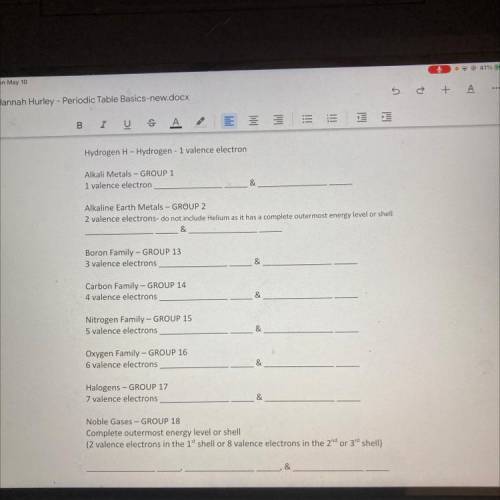
Hydrogen H-Hydrogen - 1 valence electron
Alkali Metals - GROUP 1
1 valence electron
Al...

Chemistry, 10.05.2021 23:00 gujaratif932
Hydrogen H-Hydrogen - 1 valence electron
Alkali Metals - GROUP 1
1 valence electron
Alkaline Earth Metals - GROUP 2
2 valence electrons- do not include Helium as it has a complete outermost energy level or shell
&
Boron Family - GROUP 13
3 valence electrons
Carbon Family - GROUP 14
4 valence electrons
Nitrogen Family - GROUP 15
5 valence electrons
&
Oxygen Family - GROUP 16
6 valence electrons
&
Halogens - GROUP 17
7 valence electrons
Noble Gases - GROUP 18
Complete outermost energy level or shell
(2 valence electrons in the 1" shell or 8 valence electrons in the 2nd or 3rd shell)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

You know the right answer?
Questions in other subjects:

Mathematics, 02.05.2021 20:30

Mathematics, 02.05.2021 20:30

Mathematics, 02.05.2021 20:30






Computers and Technology, 02.05.2021 20:30




