
Chemistry, 10.05.2021 21:30 adreyan3479
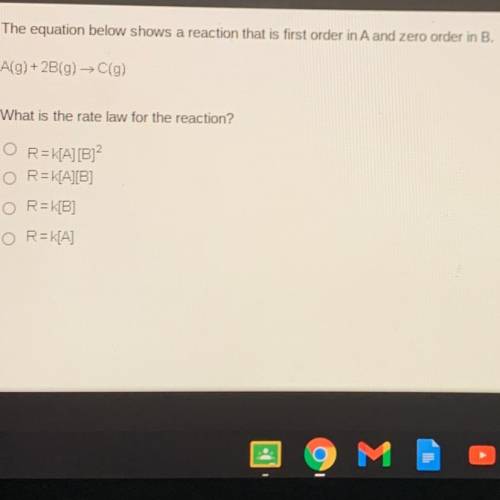
The equation below shows a reaction that is first order in A and zero order in B.
A(g) + 2B(g) →C(g)
What is the rate law for the reaction?
R=K[A] [B]2
R=K[A][B]
R=K[B]
R=K[A]

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1



Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
The equation below shows a reaction that is first order in A and zero order in B.
A(g) + 2B(g) →C(...
Questions in other subjects:

Mathematics, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01

History, 26.06.2020 15:01

English, 26.06.2020 15:01








