
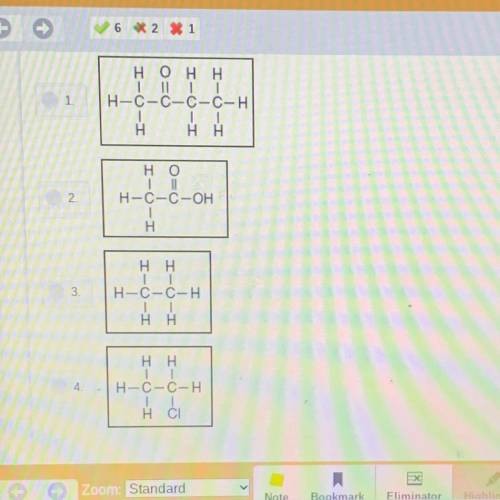
The candle is lit and dilute ethanoic acid is poured down the inside of the beaker. As the acid reacts with the baking soda, bubbles of CO2 gas form. After a few seconds
the air in the beaker is replaced by 0.20 liter of CO2 gas, causing candle flame to go out. The density of CO2 gas is 1.8 grams per liter at room temperature.
Choose the correct structural formula for the acid that was poured into the beaker.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, cynthiagutierrez65
Where can i find naap lab answers sheet/key?
Answers: 1

Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2


You know the right answer?
The candle is lit and dilute ethanoic acid is poured down the inside of the beaker. As the acid reac...
Questions in other subjects:




Mathematics, 01.07.2020 15:01


Business, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

English, 01.07.2020 15:01





