C(s) +202(g) → CO(9)

Chemistry, 09.05.2021 21:30 YoEsMyles3115
HELPP PLS!
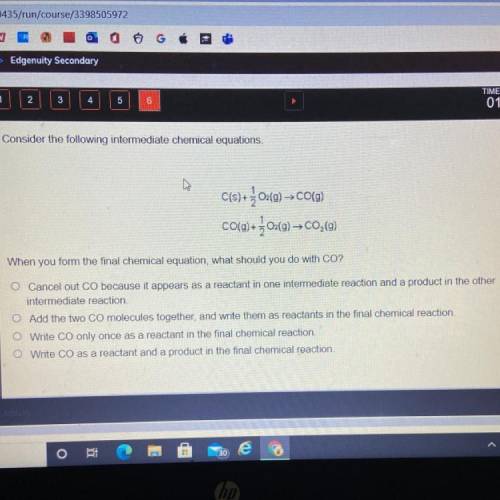
Consider the following intermediate chemical equations.
C(s) +202(g) → CO(9)
CO(g) + 304(9)–C0,(9)
When you form the final chemical equation, what should you do with CO?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, porkhappycom
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
HELPP PLS!
Consider the following intermediate chemical equations.
C(s) +202(g) → CO(9)
C(s) +202(g) → CO(9)
Questions in other subjects:


Mathematics, 13.01.2021 18:20


Mathematics, 13.01.2021 18:20

Mathematics, 13.01.2021 18:20

Chemistry, 13.01.2021 18:20

Chemistry, 13.01.2021 18:20

English, 13.01.2021 18:20


Physics, 13.01.2021 18:20



