Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 23.06.2019 00:30, rose888829
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
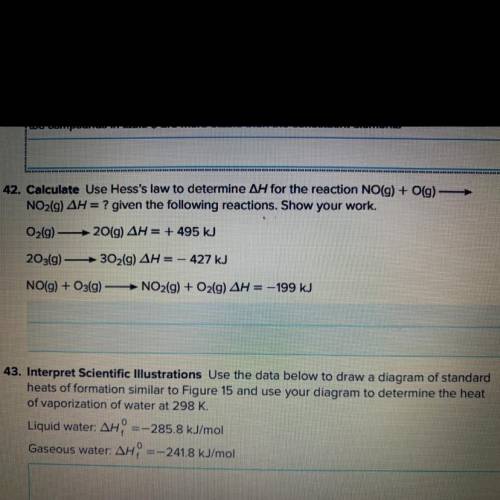
Calculate, use hess’s law to determine /\H for the reaction NO(g) + O(g)—> NO2(g) /\H=? given the...
Questions in other subjects:

Social Studies, 20.10.2021 02:20

Mathematics, 20.10.2021 02:20

Chemistry, 20.10.2021 02:20

Medicine, 20.10.2021 02:20

English, 20.10.2021 02:20



Social Studies, 20.10.2021 02:20