
Chemistry, 08.05.2021 02:00 zchwilke2981
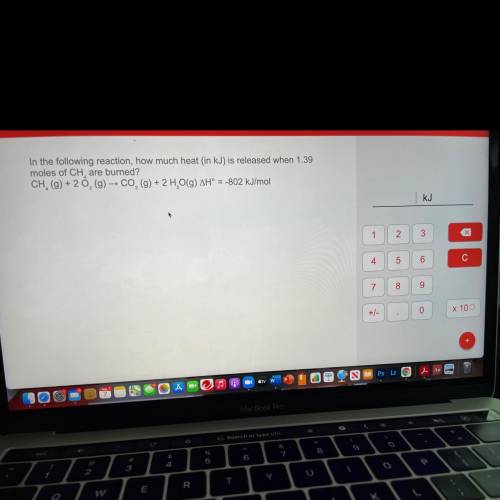
In the following reaction, how much heat (in kJ) is released when 1.39
moles of CH are burned?
CH, (g) + 20, (g) → CO2 (g) + 2 H, O(9) AH° = -802 kJ/mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
In the following reaction, how much heat (in kJ) is released when 1.39
moles of CH are burned?
Questions in other subjects:




History, 01.03.2021 21:50

Biology, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50


Spanish, 01.03.2021 21:50


Social Studies, 01.03.2021 21:50



